Ammonia concentration can reach extremely high levels in wastewater due to digestion of nitrogen-containing organic waste. Activities including discharge of wastewater and agricultural runoff can lead to contamination and excessive ammonia concentrations in natural waters. High levels of ammonia are toxic to aquatic life, and therefore monitoring the presence of ammonia and controlling the amount entering a water system is essential.
When ammonia (NH3) is dissolved in water, a portion of it will protonate to form the charged species ammonium (NH4+). Ammonia nitrogen refers to both ammonia and ammonium. The ratio of ammonia to ammonium will depend upon both the pH and temperature of the water.
pH is a measure of the concentration of positively charged hydrogen ions (H+) in water. A solution containing a high concentration of H+ ions is more acidic and has a lower pH value. The lower the pH and the warmer the water, the higher the concentration of ammonium due to the increase of H+ ions.
Different biological processes respond differently to each form of ammonia nitrogen. Ammonium will diffuse through organic membranes more slowly than ammonia and is therefore significantly less toxic to aquatic life.
High ammonia concentrations in water can also have detrimental effects to aquatic life due to a process known as eutrophication. Excessive concentrations of nitrogen, along with other chemical parameters including phosphates, can lead to high levels of aquatic plant growth and algal blooms which cover the surface of the water. The algae block sunlight and use up all the dissolved oxygen in the water so aquatic life can no longer survive.
Due to the reasons discussed above, the removal of ammonia has become a requirement and part of the process for the treatment of wastewater and sewage. Nitrification is a 2-step biological process where ammonia is removed from the wastewater and converted to nitrate using aerobic bacteria. The first step of nitrification involves oxidation of ammonia to nitrite, and then the second step uses nitrite-oxidising bacteria to oxidise nitrite to nitrate. This process is shown in the schematic below.
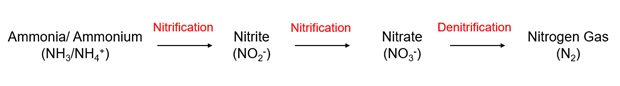
Some environmental protection standards require the nitrate resulting from the nitrification process to also be removed. In this case, an additional denitrification step can be added. This uses denitrification bacteria to convert the nitrate to nitrogen gas which is harmless to the environment.
Photometry is one of the most common methods for measuring ammonia in environmental and wastewater applications. There are two main methods to choose between when measuring ammonia using photometry: the Nessler method and the Indophenol method.
The Nessler method is usually the most popular method for ammonia testing in wastewater applications. This method involves the use of Nessler’s reagent. In the presence of ammonia, a yellow compound is formed which is proportional to the amount of ammonia present in the sample. The Nessler method is widely used in industry, but it does require the use of mercury which is hazardous and therefore can be difficult to process, transport and dispose of.
The alternative option for ammonia measurement is the Indophenol method. For low concentrations of ammonia, this is the most accurate and precise method of measurement. Using the Indophenol method, ammonia reacts with an indicator through a series of reactions, causing a yellow solution to turn green to blue in the presence of ammonia.
Both Nessler and Indophenol reagents are available from Palintest, covering ranges from 0–1 mg/L to 0–100 mg/L with multiple options in between. All ammonia reagent options are compatible with our Lumiso Ammonia handheld photometer, allowing flexibility with testing options.

Palintest’s Lumiso Ammonia Photometer
The Lumiso Ammonia Photometer is a lightweight, handheld photometer capable of measuring ammonia using both Nessler and Indophenol methods with multiple ranges between 0–100 mg/L.
Because recording results that are traceable and auditable is of high importance when monitoring effluents and environmental water, the Lumiso Ammonia can store up to 50 data sets on the instrument’s memory and has a live clock so the exact date and time for each measurement is saved. These results can also be transferred via QR code or USB to another device to record or share as required.
Another important factor, especially when testing in-field, is obtaining accurate and reliable measurements easily. Lumiso Ammonia has been designed with photometer performance and ease-of-use in mind. The optical chamber of the Lumiso instrument is streamlined, removing dirt traps and tough-to-clean areas so the optics are easy to clean and minimum maintenance is required. This saves time on instrument maintenance and ensures performance accuracy is maintained.
The Lumiso Ammonia kit includes the following design features so obtaining accurate and reliable ammonia measurements is as simple as possible:

- Lightweight, handheld photometer which can be used to measure ammonia using both Nessler and Indophenol reagents with a wide choice of test ranges.
- A reusable reagent box to store any reagents purchased with the kit.
- Easy-to-follow pictorial instructions to ensure instrument set up and use is easy.
- Individual slots for accessories such as Tubetest reagents and the adapter required for Tubetest measurement.
- A hard case so everything is easily stored and protected during transport.
For more information about ammonia testing options and our Lumiso Ammonia photometer, please contact a member of our team by clicking here.



